Amid the gloom and fear of Covid-19, there is a ray of hope. A coronavirus vaccine developed by the University of Oxford has been found to be safe and initial reports suggest that it triggers an immune response.
But before you start celebrating, a word of caution. These are initial days and there is a long way to go.
Researchers at Oxford admit that “There is still much work to be done before we can confirm if our vaccine will help manage the Covid-19 pandemic, but these early results hold promise.”
The results so far are promising, but their main purpose is to ensure the vaccine is safe enough to give to people.
The trial has also been expanded to other countries because levels of coronavirus are low in the UK, making it hard to know if the vaccine is effective.
There will be a large trial involving 30,000 people in the US as well 2,000 in South Africa and 5,000 in Brazil.
There are also calls to perform “challenge trials” in which vaccinated people are deliberately infected with coronavirus. However, there are ethical concerns due to a lack of treatments.
India also has promising vaccines on trial. Human trials of the indigenously developed coronavirus vaccine called COVAXIN began today, AIIMS-Delhi Director Dr Randeep Guleria announced today. It would take at least three months for researchers to arrive at the first set of data, he added.

Phase 1 of the three-phase human trials started as coronavirus cases in India crossed the 11 lakh-mark with 40,000 fresh cases of Covid-19.
“It (starting trials) is heartening because it’s an indigenous vaccine; making a new vaccine is an achievement. Even if a vaccine is first developed somewhere else in the world, India will be mass-producing it. We are good at it,” he said.
Some 1,125 healthy volunteers will be injected with inactivated Sars-CoV-2 so their body can produce COVID-fighting antibodies.
Phase-1 includes 375 volunteers in the 18-55 age group with no co-morbidity; phase 2 will have 750 individuals in the 12-65 age group and Phase 3 will be done with a larger population.
The Oxford trials involved 1,077 people and the vaccine led to the trial group develop antibodies and T-cells that can fight coronavirus.
This is only a hope as of now as the trial group is too small. It is not known if the vaccine can offer protection and larger trials are under way.
More than 10,000 people will take part in the next stage of the trials in the UK.
The UK has already ordered 100 million doses of the vaccine.
The new vaccine is called ChAdOx1 nCoV-19 and is being fast-tracked. It is made from a genetically engineered virus that causes the common cold in chimpanzees.

The genetically engineered virus has been drastically modified. It cannot cause infections in people and also to make it “look” more like coronavirus.
Scientists did this by transferring the genetic instructions for the coronavirus’s “spike protein.” This is the protein that the virus uses to invade our cells.
In other words, the vaccine resembles the coronavirus and the immune system can learn how to attack it.
World over, the focus on coronavirus has been about antibodies. However, these are only one part of our immune defence.
Antibodies are small proteins made by the immune system that stick onto the surface of viruses. Neutralising antibodies can disable the coronavirus.
T-cells are a type of white blood cell that help co-ordinate the immune system and are able to spot which of the body’s cells have been infected and destroy them.
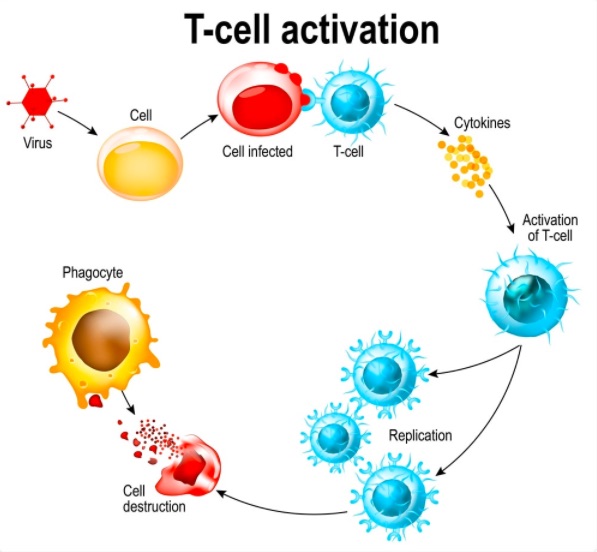
Nearly all effective vaccines induce both an antibody and a T-cell response.
Levels of T-cells peaked 14 days after vaccination and antibody levels peaked after 28 days. The study has not run for long enough to understand how long they may last, the study in ‘The Lancet‘ showed.
NEW—UK’s #COVID19 vaccine is safe and induces an immune reaction, according to preliminary results https://t.co/rDPlB9fDKr pic.twitter.com/z2t9Aubjim
— The Lancet (@TheLancet) July 20, 2020
But the key question everyone wants to know is does the vaccine work, does it offer protection.
The study showed 90% of people developed neutralising antibodies after one dose. Only ten people were given two doses and all of them produced neutralising antibodies.
The vaccine is reportedly safe, but there are side-effects. Nearly 70% of the people on the trial developed either fever or headache. The researchers say this is not dangerous and could be managed with paracetamol.